Associate Clinical Programmer Jobs at Johnson & Johnson in India for SDTM programming, SAS skills, hybrid work, full-time role.
About the Company
Johnson & Johnson is one of the world’s most trusted and innovative healthcare companies, with a legacy spanning more than 135 years. Guided by its core belief that health is everything, Johnson & Johnson continuously strives to improve human health through cutting-edge research, innovation, and ethical practices. Associate Clinical Programmer Jobs
The company operates across two major segments: Innovative Medicine and MedTech, delivering life-changing solutions that prevent, treat, and cure complex diseases. Johnson & Johnson’s global presence, strong research foundation, and commitment to patients make it a leader in pharmaceutical development, medical devices, and healthcare technology.
In India, Johnson & Johnson has established itself as a preferred employer in the clinical research, biostatistics, and data analytics domains. The organization offers a collaborative work culture, global exposure, continuous learning opportunities, and the chance to contribute to meaningful healthcare outcomes that impact millions worldwide. Associate Clinical Programmer Jobs
By joining Johnson & Johnson, professionals become part of a mission-driven organization focused on innovation, integrity, and excellence in healthcare delivery. Associate Clinical Programmer Jobs
Job Details
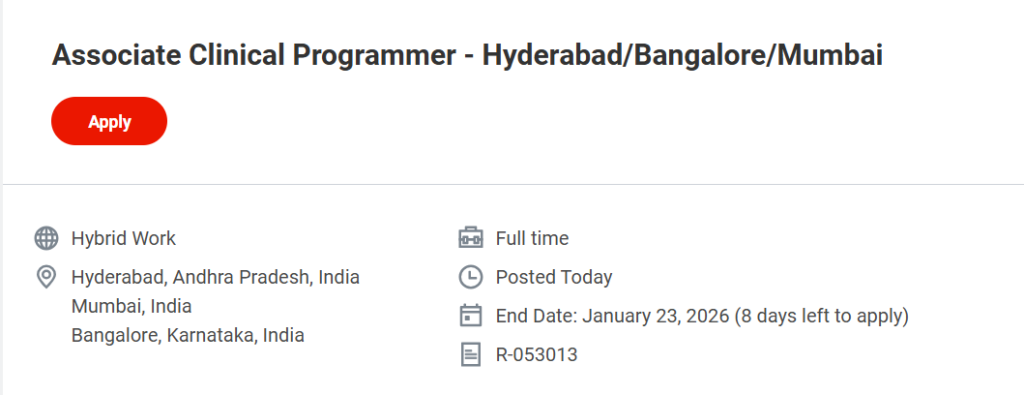
- Job Title: Associate Clinical Programmer
- Job Requisition ID: R-053013
- Job Function: Data Analytics & Computational Sciences
- Job Sub Function: Biostatistics
- Job Category: Scientific / Technology
- Employment Type: Full Time
- Work Mode: Hybrid Work
- Job Locations:
- Hyderabad, Andhra Pradesh, India
- Bangalore, Karnataka, India
- Mumbai, India
- Application Deadline: January 23, 2026
Job Description
Johnson & Johnson is hiring Associate Clinical Programmers to support clinical programming activities, with a focus on SDTM programming, data analysis, and clinical reporting. This role is ideal for early-career professionals with foundational programming knowledge who are looking to build a strong career in clinical data programming and biostatistics. Associate Clinical Programmer Jobs
The Associate Clinical Programmer is an individual contributor role, responsible for supporting low-complexity clinical trial programming tasks under guidance from senior programmers and project leads. The role involves working with structured clinical data, supporting data cleaning, ensuring compliance with CDISC standards, and contributing to high-quality clinical trial deliverables. Associate Clinical Programmer Jobs
Candidates will be part of a collaborative programming team and will gain hands-on experience in clinical trials, regulatory submissions, and cross-functional project support within a global pharmaceutical environment.
Key Responsibilities
- Perform clinical trial programming activities of low complexity while ensuring accuracy, quality, and timely delivery.
- Design, develop, and maintain programs to support clinical data analysis and reporting.
- Support SDTM and non-standard data package creation, verification, and delivery for clinical studies and regulatory submissions.
- Execute programming activities in alignment with departmental SOPs, processes, and quality standards.
- Conduct quality control checks, validation, and verification of outputs to support clinical analyses.
- Review project specifications, requirements, and documentation, providing constructive feedback where required.
- Collaborate effectively with biostatisticians, data managers, medical monitors, and cross-functional teams.
- Support data cleaning activities by programming edit checks, review listings, and data visualizations.
- Assist in data reporting by creating listings, tables, and visual outputs for medical and central monitoring.
- Ensure compliance with Good Clinical Practice (GCP), data privacy standards, and internal training requirements.
- Maintain accurate time reporting and adhere to business and operational processes.
- Contribute to process improvement initiatives and departmental innovation projects when opportunities arise.
Associate Clinical Programmer Jobs
Skills / Qualifications
Educational Qualifications:
- Bachelor’s degree or higher in one of the following disciplines:
- Computer Science
- Mathematics
- Statistics
- Data Science / Data Analytics / Data Engineering
- Public Health
- Life Sciences or other relevant scientific or technical fields
- Equivalent theoretical or technical expertise will also be considered.
Required Technical Skills:
- Basic understanding of data structures and programming concepts.
- Working knowledge of programming languages used in clinical and statistical programming, such as:
- SAS (preferred)
- R
- Python
- Familiarity with clinical trial data and reporting workflows is an advantage.
- Understanding of CDISC standards (SDTM knowledge preferred).
- Basic knowledge of statistical programming and data manipulation techniques.
Associate Clinical Programmer Jobs
Soft Skills and Competencies:
- Strong analytical and problem-solving skills.
- Good written and verbal communication abilities.
- Ability to work effectively in a team-based environment.
- Detail-oriented mindset with a focus on data accuracy and compliance.
- Willingness to learn and adapt in a regulated clinical research setting.
Associate Clinical Programmer Jobs
Preferred Skills
- Statistical Analysis Systems (SAS) Programming
- Biostatistics and Statistical Concepts
- Data Modeling and Advanced Analytics
- Understanding of Biological and Life Sciences
- Knowledge of Data Privacy Standards
- Familiarity with Research Ethics and GCP
- Report Writing and Documentation Skills
- Analytical Reasoning and Execution Focus
- Technological Awareness and Data Savviness
- Project Support Experience in Clinical Research
Benefits / Perks
Johnson & Johnson offers a comprehensive and competitive benefits package designed to support employee growth, wellbeing, and work-life balance:
- Hybrid work model offering flexibility and productivity balance
- Opportunity to work on global clinical trials and regulatory submissions
- Structured learning and career development programs
- Exposure to advanced analytics and clinical programming technologies
- Collaborative and inclusive work environment
- Competitive salary and performance-based rewards
- Strong focus on employee wellbeing and ethical workplace practices
- Long-term career growth within a globally recognized healthcare organization
Associate Clinical Programmer Jobs
Why You Should Join Johnson & Johnson
Joining Johnson & Johnson as an Associate Clinical Programmer provides a unique opportunity to begin or strengthen your career in clinical programming and biostatistics within a world-class organization.
You will gain exposure to global clinical development programs, work alongside experienced professionals, and contribute to healthcare innovations that make a real difference in patients’ lives. The company’s strong emphasis on training, compliance, quality, and innovation ensures continuous professional development. Associate Clinical Programmer Jobs
If you are passionate about data, clinical research, and improving healthcare outcomes, Johnson & Johnson offers a stable, ethical, and growth-oriented environment to build a successful long-term career. Associate Clinical Programmer Jobs
How to Apply
Interested and eligible candidates can apply online through the official Johnson & Johnson careers portal.
Early application is recommended as positions may be filled before the deadline. Associate Clinical Programmer Jobs
FAQs
Q1. Is this a remote job?
This role follows a hybrid work model, combining office and remote work as per company policy.
Q2. Is SAS mandatory for this role?
SAS knowledge is preferred, especially for clinical programming, but candidates with basic exposure to R or Python may also be considered.
Q3. Are freshers eligible to apply?
This role is suitable for early-career professionals with foundational programming knowledge. Relevant internships or academic experience can be beneficial.
Q4. What clinical standards are involved in this role?
The role involves working with CDISC standards, particularly SDTM, along with compliance to GCP and data privacy guidelines.
Q5. What is the last date to apply?
The application deadline is January 23, 2026.
For verified pharma job updates, visit Mypharmajob.com – India’s trusted pharma career portal.
Note: This job information is sourced from publicly available official sources. We share it for reference purposes only and do not claim ownership of the job posting.


